

Permeabilidad inigualable de 1 año y libertad de TLR de 3 años
El Stent Supera™ se ha estudiado en más de 2.000 pacientes de todo el mundo en el ensayo SUPERB y en 16 estudios retrospectivos. En particular, en los 17 estudios, el stent periférico Supera™ mostró resultados duraderos con cero fracturas a 1 año.1,4-19
Ensayo SUPERB
A 1 año, el stent Supera™ demostró una permeabilidad primaria del 91% cuando se desplegó nominalmente*. A los 3 años, la ausencia de revascularización de lesiones dirigidas (TLR) fue del 94% cuando se desplegó nominalmente.*,1
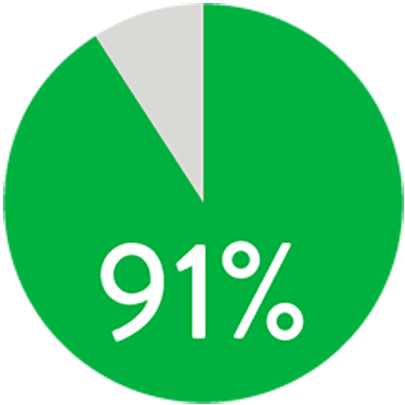
PERMEABILIDAD (K-M) A 1 AÑO
Cuando se despliega nominalmente*
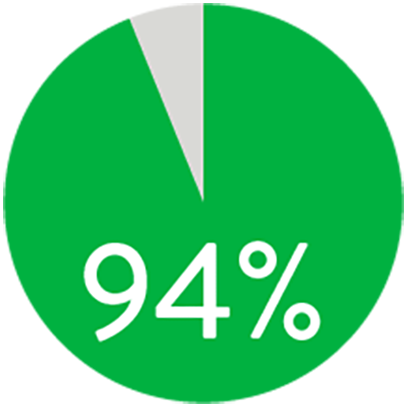
LIBERTAD DE TLR A LOS 3 AÑOS
Cuando se despliega nominalmente*
*El despliegue nominal se define como la longitud del stent en el momento del despliegue que se encuentra dentro de +/- 10% de la longitud del stent. Estos datos provienen de un análisis post-hoc no potenciado. K-M = Kaplan Meier.
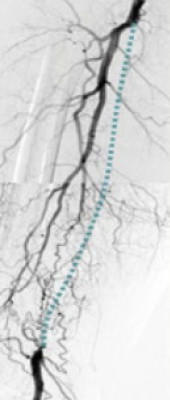
Permeabilidad constante independientemente de la longitud de la lesión
Con algunos stents periféricos, el aumento de la longitud de las lesiones puede conducir a una disminución de las tasas de permeabilidad.20 El stent Supera™ se distingue por sus tasas de permeabilidad consistentemente altas en lesiones que abarcan longitudes de 5,3 cm a 28,0 cm.†
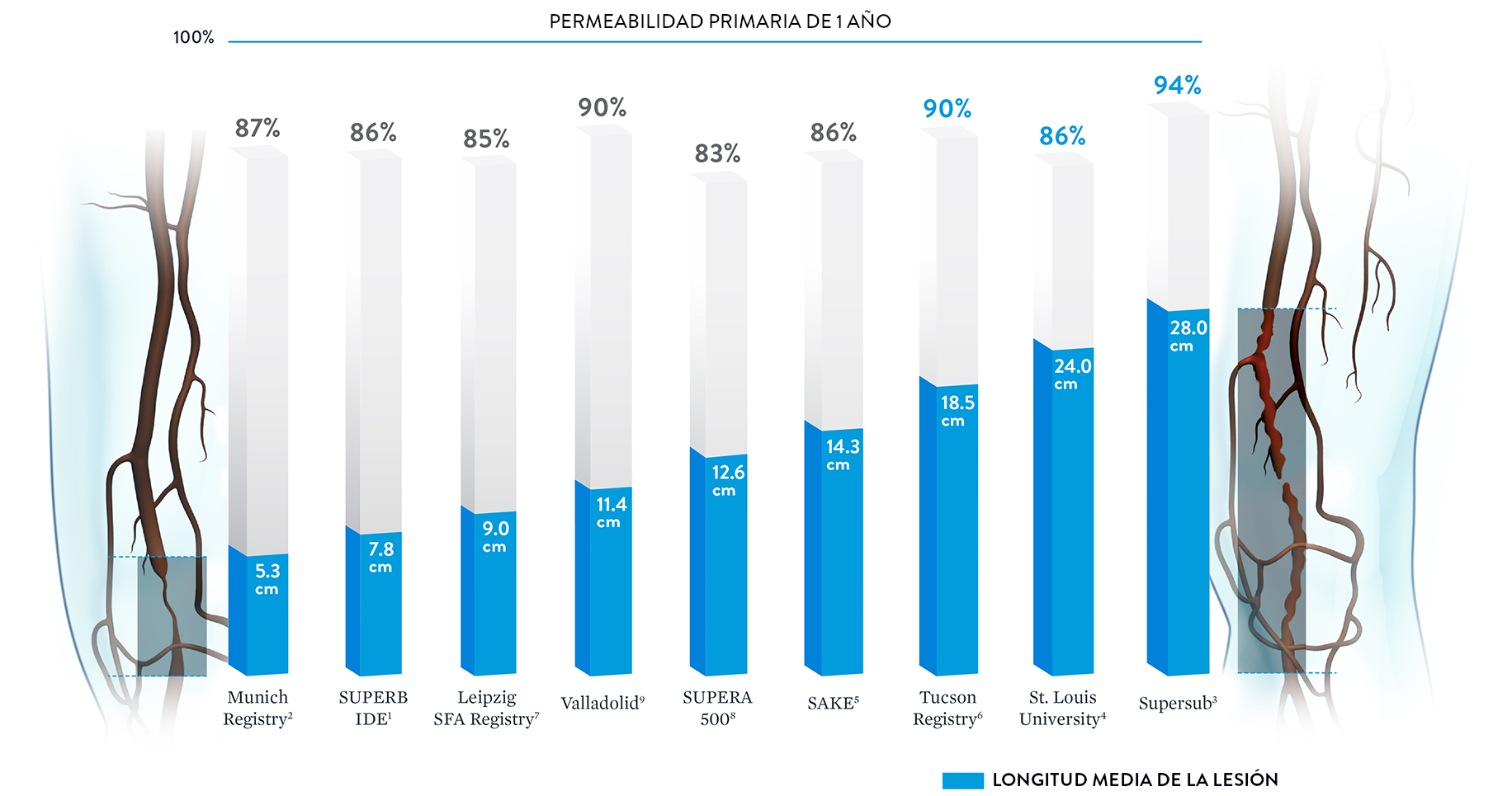
† Se incluyeron los datos publicados si se disponía de la longitud y la permeabilidad de la lesión.
Nota: Los resultados de diferentes ensayos clínicos no son directamente comparables. Información proporcionada únicamente con fines educativos.
Excelentes resultados desde lesiones simples hasta lesiones complejas
Ya sea que se trate de lesiones simples (TASC A & B) o complejas (TASC C & D), el stent Supera™ se asocia con datos de rendimiento de permeabilidad alta y consistentes a 1 año.1-4
| Simple | |||
|---|---|---|---|
 |
Ensayo/Estudio | REGISTRO DE MÚNICH2 | SUPERB1 |
| Longitud de la lesión | 5.3cm | 7.8cm | |
| Lesiones TASC A&B | 100% | 94% | |
| 1 año Permeabilidad |
86.7% | 90.5% | |
| Sitios | Centro Único | Multicéntrico (46 sítios) | |
| # Pacientes | 70 | 264 | |
| Complejo | |||
|---|---|---|---|
 |
Ensayo/Estudio | ST. LOUIS4 | SUPERSUB3 |
| Longitud de la lesión | 24cm | 28cm | |
| Lesiones TASC C&D | 78% | 100% | |
| CTOs | Desconocido | 100% | |
| 1 año Permeabilidad |
85.6% | 94.1% | |
| Sitios | Centro Único | Centro Único | |
| # Pacientes | 48 | 34 | |

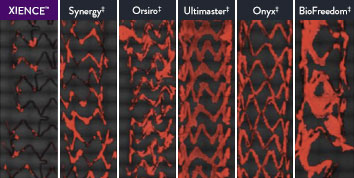
The XIENCE™ Stent is also recognized as being significantly more anti-thrombotic than other DES on the market. As shown in the study findings, XIENCE™ Stent reveals significantly less (p < 0.01) platelet adhesion—shown in red in the confocal microscopy images—than other DES, and platelet adhesion is an important factor in stent thrombosis.*8 These findings suggest that this stent choice “may be ideally suited for very short-term DAPT.”8
*Ex Vivo Swine Shunt Model.

Diamondback 360™ OAS Gives You the Versatility to Treat Challenging Cases
Treat even the most severely calcified lesions, with under 2-minute setup7,8 and predictable procedure times.2
Facilitates antegrade and retrograde treatment of:
- Long, Diffuse Lesions
Successfully treated lesions up to 60 mm in length in real-world study.9 - Heavily Stenosed Lesions
Crossed >99% of lesions with <2% pre-dilatation in the ORBIT II study.1,10 - Nodular Lesions
Effectively treats nodular calcification.5,6 - Ostial Lesions
Safely treats ostial lesions.11-13
Low Profile
6F compatible for femoral or radial access.14
Multiple Vessel Sizes
A single 1.25 mm crown treats vessels 2.5 mm to 4.0mm14
|
|
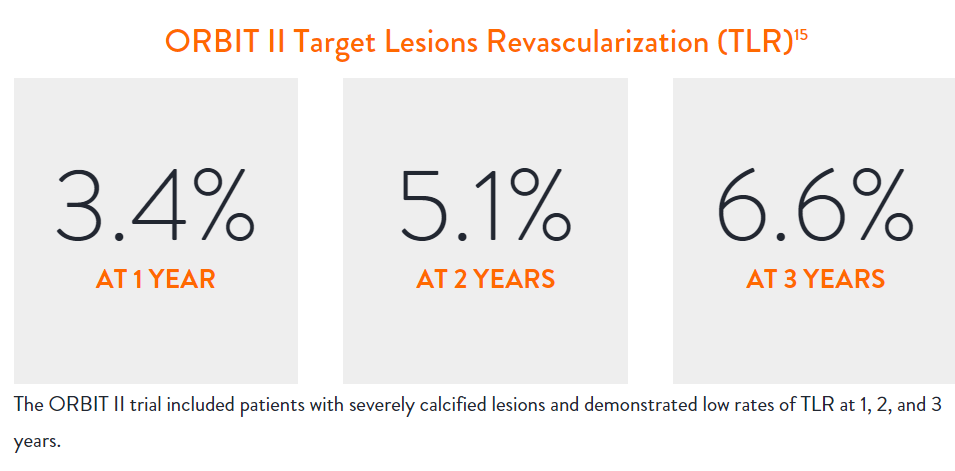
Proven Safety |
Procedural Success |
Low Q-Wave MI Rate |
>2,200 |
<1% |
97.7% |
0.9% |
|
Patients Across 11 |
Component Angiographic |
Crossing and Stent |
In the ORBIT II |
Sustained Clinical Performance
Data are for ORBIT II TLR in the OA+DES patient cohort.

“Stopping DAPT at 3 months in selected patients after [XIENCE™ Stent] implantation was at least as safe as the prolonged DAPT regimen adopted in the historical control group.”
— Masahiro Natsuaki, MD, STOPDAPT Trial9

STOPDAPT 2 Trial Design and Randomization10

Short 1-Month DAPT
- 0 to 1-month: Aspirin + P2Y12
- After 1-month: Clopidogrel monotherapy

12-Month DAPT
- 0 to 1-month: Aspirin + P2Y12
- 1 to 12-month: Aspirin + Clopidogrel
- 12 to 60-month: Aspirin monotherapy

- Successful PCI using CoCr everolimus-eluting stent: XIENCE™
- Eligible for DAPT (aspirin/P2Y12 receptor blocker) for 1 year

- Patients who need oral anticoagulants
- History of intracranial bleeding
- Major in-hospital complications (MI/stroke/major bleeding)
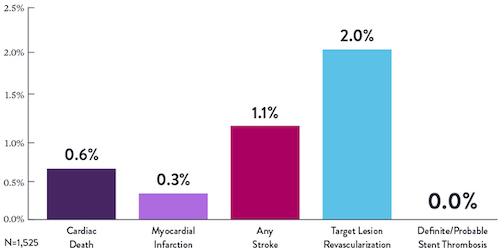
STOPDAPT Study: XIENCE™ Stent with 3-Month DAPT Is Feasible9
STOPDAPT9 was the first prospective trial to study DAPT cessation at 3 months after implantation. Among other 1-year outcomes, the XIENCE™ Stent rate of stent thrombosis was 0.0%.
STOPDAPT Study Demonstrates Feasibility of XIENCE™ Stent with 3-Month DAPT9

Learn more about STOPDAPT 2

“It was noteworthy that no definite or probable stent thrombosis occurred in [XIENCE™ Stent] patients enrolled in STOPDAPT.”
— Masahiro Natsuaki, MD, STOPDAPT Trial9

STOPDAPT-3 Trial Design and Randomization11

- PCI with planned exclusive use of CoCr-EES (XIENCE)
- ACS presentation or ARC-HBR
- Eligible for DAPT (Aspirin/P2Y12inhibitor) for 1 month
Study design and Randomization
Group 1:
0 to 1-month: Aspirin + P2Y12 (Prasugrel)
After 1-month: Clopidogrel monotherapy
Group 2:
0 to 1-month: P2Y12 (Prasugrel)
After 1-month: Clopidogrel monotherapy
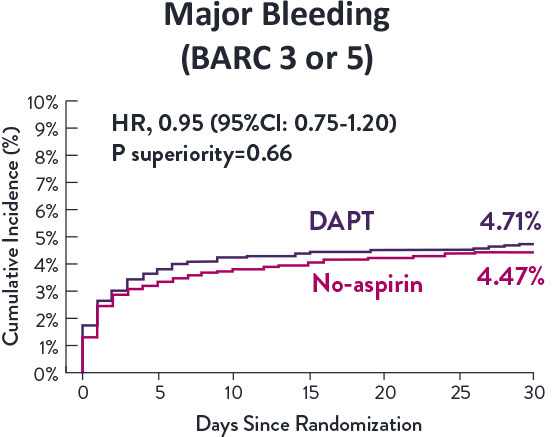
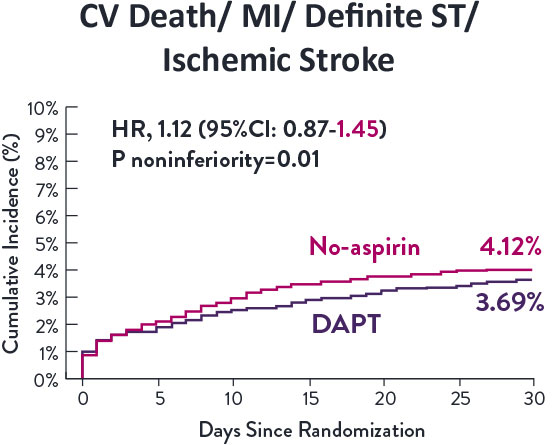
STOPDAPT-3 Trial 11 was designed to explore 0-month DAPT* (SAPT˄ using only P2Y12 inhibitor) for ACS and HBR patients.
Though the results are comparable for both bleeding and ischemic events for DAPT and SAPT arms, the study did not meet its endpoint and concluded to use DAPT for 1 month after PCI.

XIENCE™ Stent remains the ONLY DES with the shortest DAPT indication, as short as 28 days.12


Referencias
- Garcia L. et al., Catheterization and Cardiovascular Interventions 2017 Jun 1;89(7):1259-1267.
- Treitl, K.M., et al. European Radiology.2017; 10.1007.
- Palena L.M. et al. Catheterization and Cardiovascular Intervention.2016.
- Brescia AA. et al., J Vasc Surg. 2015 Jun;61(6):1472-8
- George JC. et al., J Vasc Interv Radiol. 2014 Jun;25(6):954-61.
- Montero-Baker M. et al., J Vasc Surg. 2016 Oct;64(4):1002-8.
- Scheinert D. et al., J Endovasc Ther. 2011 Dec;18(6):745-52.
- Werner M. et al., EuroIntervention. 2014 Nov;10(7):861-8.
- San Norberto EM. et al., Ann Vasc Surg. 2017 May;41:186-195.
- Chan YC. et al., J Vasc Surg. 2015 Nov;62(5):1201-9.
- Dumantepe M. Vasc Endovascular Surg. 2017 Jul;51(5):240-246.
- Goltz JP. et al., J Endovasc Ther. 2012 Jun;19(3):450-6.
- León LR Jr. et al., J Vasc Surg. 2013 Apr;57(4):1014-22.
- Myint M. et al., J Endovasc Ther. 2016 Jun;23(3):433-41.
- Palena LM. et al., J Endovasc Ther. 2018 Oct;25(5):588-591.
- Scheinert D. et al., JACC Cardiovasc Interv. 2013 Jan;6(1):65-71.
- Steiner S. et al., J Endovasc Ther. 2016 Apr;23(2):347-55.
- Teymen B. et al., Vascular. 2018 Feb;26(1):54-61.
- Bhatt H. et al., Cardiovasc Revasc Med. 2018 Jul;19(5 Pt A):512-515.
- Shroë H. Superficial femoral artery PTA or stenting? 5-Year results. CIRSE 2011; Munich, Germany

MAT-XXXXXXX



