

Catéter de Imagen Dragonfly Opstar™: El nuevo diseño incrementa la confianza en su capacidad de entrega.
El Catéter de Imagen Dragonfly OpStar™ se utiliza para obtener imágenes intravasculares por medio de Tomografía de Coherencia Óptica (OCT). El Catéter de Imagen Dragonfly OpStar™ es compatible con OPTIS™ Next System (software Ultreon™ 1.0), OPTIS™ Mobile System (software AptiVue™ versión 5.1 o superior) e ILUMIEN™ System (software AptiVue™ versión D3 o superior) ¹.
- Navegar por la anatomía tortuosa y acceder a las lesiones distales.
- Proporciona imágenes más brillantes y de mayor calidad. ²
 |
 |
 |
Capacidad de entrega segura
|
CAPACIDAD DE CRUCE
MEJORADA • Perfil de punta bajo
• Perfil de cruce 26% más bajo² |
EMPUJE
MEJORADO • Capaz de resistir un
47% mas de fuerza proximal
sin prolapsar.²
|
TRAZABILIDAD MEJORADA • Puerto reforzado • Puerto de RX de la guía sin inclinación • Capa interior lubricante para una menor interacción con el alambre guía² |
| Dragonfly OpStar™ Catéter de imagen |
 |
Decisiones de Tratamiento Precisas
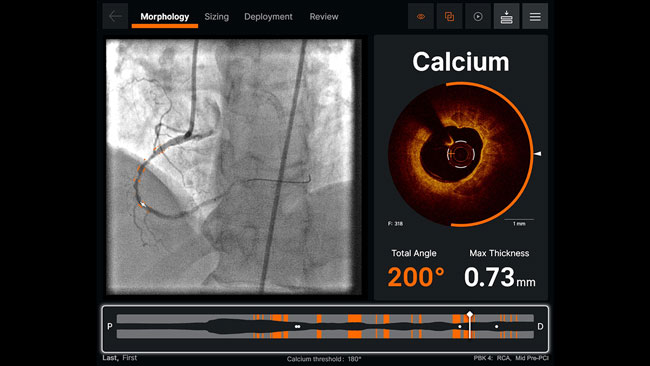
NUEVO El rediseño de la lente proporciona imágenes 44% más brillantes, para ayudar a mejorar la identificación de la lamina elástica externa (EEL) y la morfología2
| Construcción actualizada para mejorar la durabilidad, el brillo y la calidad de imagen. | Mayor enfoque donde lo necesita. |
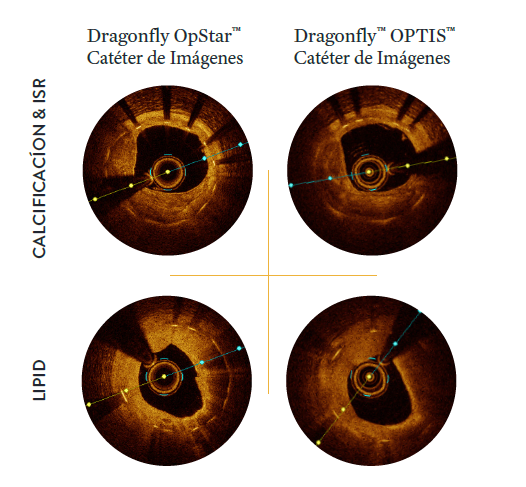
| NOTA: Imágenes adquiridas con el software AptiVue Imaging²
|
| Dragonfly OpStar™ Catéter de imagen |
 |
| Dragonfly OPTIS™ Catéter de imagen |
 |
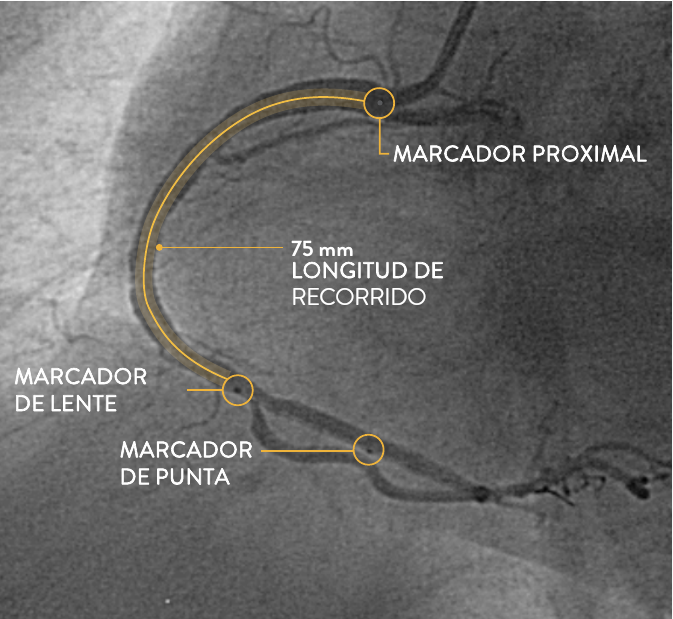
| NUEVO La ubicación del marcador proximal esta más lejos del lente de imagen para poder encuadrar un retroceso de 75 mm² |
|
| NUEVO Marcador de lente ahora inmediatamente proximal a la lente de imagen para un co-registro más preciso. Compárese con el lente del catéter de imágenes Dragonfly™ OPTIS™, que es proximal al lente por 1-2 mm² |

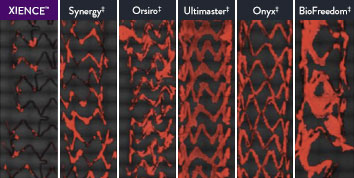
The XIENCE™ Stent is also recognized as being significantly more anti-thrombotic than other DES on the market. As shown in the study findings, XIENCE™ Stent reveals significantly less (p < 0.01) platelet adhesion—shown in red in the confocal microscopy images—than other DES, and platelet adhesion is an important factor in stent thrombosis.*8 These findings suggest that this stent choice “may be ideally suited for very short-term DAPT.”8
*Ex Vivo Swine Shunt Model.

Diamondback 360™ OAS Gives You the Versatility to Treat Challenging Cases
Treat even the most severely calcified lesions, with under 2-minute setup7,8 and predictable procedure times.2
Facilitates antegrade and retrograde treatment of:
- Long, Diffuse Lesions
Successfully treated lesions up to 60 mm in length in real-world study.9 - Heavily Stenosed Lesions
Crossed >99% of lesions with <2% pre-dilatation in the ORBIT II study.1,10 - Nodular Lesions
Effectively treats nodular calcification.5,6 - Ostial Lesions
Safely treats ostial lesions.11-13
Low Profile
6F compatible for femoral or radial access.14
Multiple Vessel Sizes
A single 1.25 mm crown treats vessels 2.5 mm to 4.0mm14
|
|
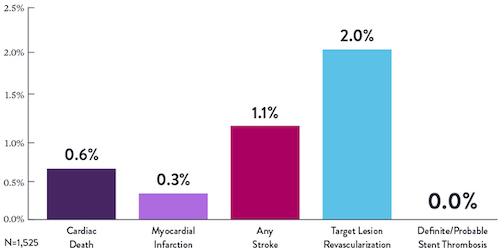
Proven Safety |
Procedural Success |
Low Q-Wave MI Rate |
>2,200 |
<1% |
97.7% |
0.9% |
|
Patients Across 11 |
Component Angiographic |
Crossing and Stent |
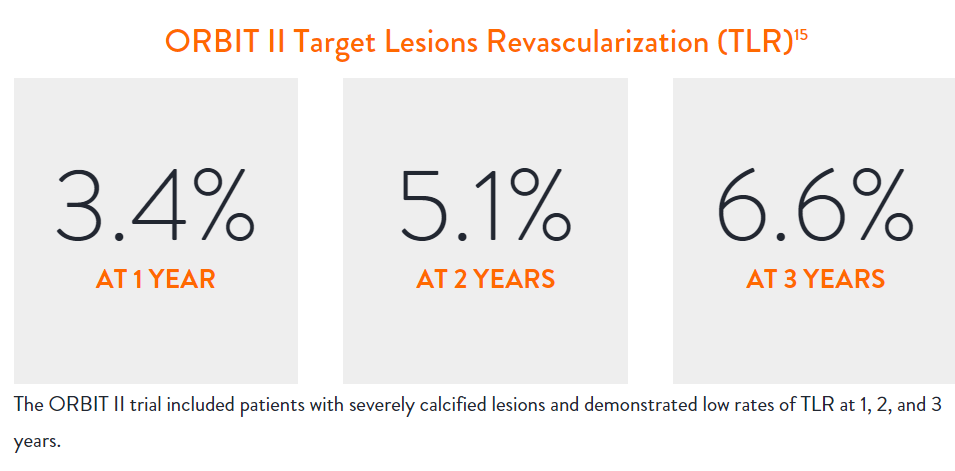
In the ORBIT II |
Sustained Clinical Performance
Data are for ORBIT II TLR in the OA+DES patient cohort.

“Stopping DAPT at 3 months in selected patients after [XIENCE™ Stent] implantation was at least as safe as the prolonged DAPT regimen adopted in the historical control group.”
— Masahiro Natsuaki, MD, STOPDAPT Trial9

STOPDAPT 2 Trial Design and Randomization10

Short 1-Month DAPT
- 0 to 1-month: Aspirin + P2Y12
- After 1-month: Clopidogrel monotherapy

12-Month DAPT
- 0 to 1-month: Aspirin + P2Y12
- 1 to 12-month: Aspirin + Clopidogrel
- 12 to 60-month: Aspirin monotherapy

- Successful PCI using CoCr everolimus-eluting stent: XIENCE™
- Eligible for DAPT (aspirin/P2Y12 receptor blocker) for 1 year

- Patients who need oral anticoagulants
- History of intracranial bleeding
- Major in-hospital complications (MI/stroke/major bleeding)
STOPDAPT Study: XIENCE™ Stent with 3-Month DAPT Is Feasible9
STOPDAPT9 was the first prospective trial to study DAPT cessation at 3 months after implantation. Among other 1-year outcomes, the XIENCE™ Stent rate of stent thrombosis was 0.0%.
STOPDAPT Study Demonstrates Feasibility of XIENCE™ Stent with 3-Month DAPT9

Learn more about STOPDAPT 2

“It was noteworthy that no definite or probable stent thrombosis occurred in [XIENCE™ Stent] patients enrolled in STOPDAPT.”
— Masahiro Natsuaki, MD, STOPDAPT Trial9

STOPDAPT-3 Trial Design and Randomization11

- PCI with planned exclusive use of CoCr-EES (XIENCE)
- ACS presentation or ARC-HBR
- Eligible for DAPT (Aspirin/P2Y12inhibitor) for 1 month
Study design and Randomization
Group 1:
0 to 1-month: Aspirin + P2Y12 (Prasugrel)
After 1-month: Clopidogrel monotherapy
Group 2:
0 to 1-month: P2Y12 (Prasugrel)
After 1-month: Clopidogrel monotherapy
STOPDAPT-3 Trial 11 was designed to explore 0-month DAPT* (SAPT˄ using only P2Y12 inhibitor) for ACS and HBR patients.
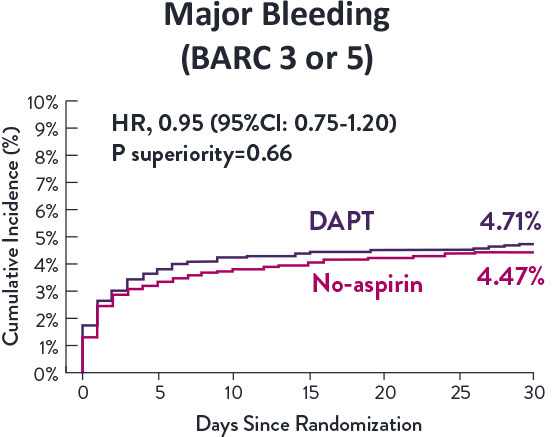
Though the results are comparable for both bleeding and ischemic events for DAPT and SAPT arms, the study did not meet its endpoint and concluded to use DAPT for 1 month after PCI.
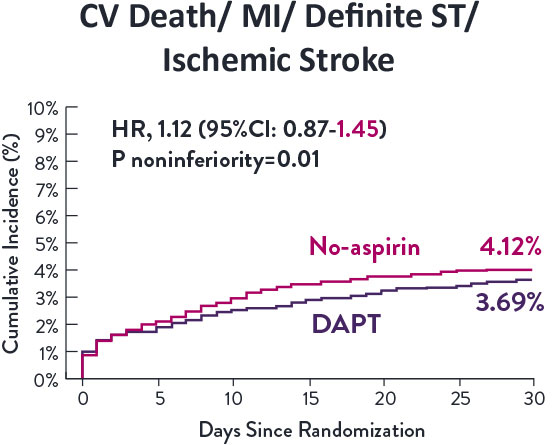

XIENCE™ Stent remains the ONLY DES with the shortest DAPT indication, as short as 28 days.12


Referencias
- Dragonfly OpStar™ Imaging Catheter Instructions for Use. Refer to IFU for additional information.
- Data on file at Abbott. Comparative claims versus the previous generation of the device, Dragonfly™ OPTIS™ Imaging Catheter.
- Dragonfly™ OPTIS™ Instructions for use (IFU). Refer to IFU for additional information.

MAT-XXXXXXX



